Despite the high response rates of chimeric antigen receptor (CAR) - T cells targeting the B-cell maturation antigen (BCMA) for multiple myeloma (MM), patients eventually still relapse. At this time there is no clear subsequent therapy. Furthermore, CAR T-cell therapy is associated with significant toxicities such as cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome.
CD70 is a normally transiently expressed on activated T-cells, B-cells, or dendritic cells, but have been found to be upregulated on many hematologic and solid cancer cells such as acute myeloid leukemia, triple negative breast cancer, and renal cell carcinoma. However, CD70 as a potential target for treatment of multiple myeloma, including patients who have progressed on BCMA targeted therapies, has not been fully investigated.
We assessed bone marrow aspirates from MM patients by flow cytometry and demonstrated that CD70 was expressed on the majority of myeloma cells, and on average, relapsed myeloma patients had higher expression of CD70. In addition, using immunohistological staining, we found that CD70 was expressed on 10 of 10 patients who had progressed on BCMA targeted therapies (seven who had progressed on belantamab mafodotin, an antibody drug conjugate, three who had progressed on idecabtagene vicleucel, a BCMA CAR-T), suggesting that CD70 could be a viable target in patients who have failed BCMA targeted therapy.
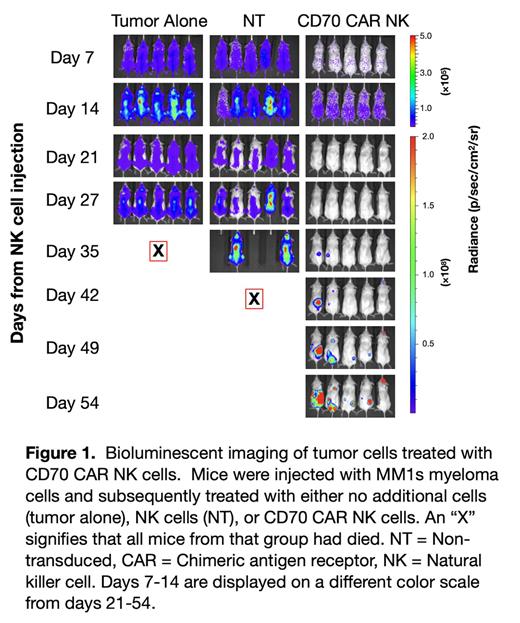
As natural killer (NK) cells are a promising off-the-shelf treatment with minimal toxicity as compared to CAR-T cells, we generated CD70-targeting CAR NK cells for the treatment of MM. We found in the chromium cytotoxicity assay that CD70 CAR NK cells had higher killing as compared to non-transduced (NT) NK cells at multiple effector-to-target ratios against MM1s, a myeloma cell line confirmed to express CD70. There was no difference in killing when co-cultured with H929, a myeloma cell line lacking CD70 expression, demonstrating the specificity of the CD70 CAR NK cell. The enhanced killing was also confirmed in an incucyte assay in which the CD70 CAR NK cells cleared tumor faster than NT NK, even in MM1s in which BCMA was knocked out using CRISPR (simulating BCMA antigen loss). When tested in a myeloma mouse model, the CD70 CAR NK exhibited greater tumor control as shown in Figure 1, when compared to NT NK, which lead to increased survival of the mice. Similar results were seen in a mouse model using BCMA KO myeloma cells.
In summary, we were able to demonstrate that CD70 can be a feasible target for the treatment of multiple myeloma, including in patients who have failed BCMA targeted therapy. Based on these results, we have initiated a Phase I/II clinical trial (NCT05092451) that is currently recruiting.
Disclosures
Lin:Takeda: Patents & Royalties, Research Funding. Acharya:Takeda: Other: S. A. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical. . Banerjee:Takeda: Patents & Royalties, Research Funding. Daher, MD:Takeda: Patents & Royalties. Basar:Takeda, Affimed GmbH: Patents & Royalties. Patel:Takeda: Consultancy; AbbVie; Allogene Therapeutics, Inc.; Arcellx; Bristol Myers Squibb/Celgene Corporation; Cellectis; Janssen Pharmaceuticals, Inc.; Nektar Therapeutic; Poseida Therapeutics; Precision BioSciences, Inc.; and Takeda Pharmaceuticals U.S.A., Inc.: Research Funding; AbbVie; Arcellx, AstraZeneca; Bristol Myers Squibb/Celgene Corporation; Caribou Science; Cellectis; Curio Bioscience; Genentech; Janssen Pharmaceuticals, Inc.; Karyopharm; Legend Biotech; Merck & Co., Inc.; Oncopeptides; Pfizer; Precision BioSciences: Consultancy. Lee:Janssen: Consultancy, Research Funding; Genentech: Consultancy; Pfizer: Consultancy; Takeda: Consultancy, Research Funding; Sanofi: Consultancy; Amgen: Research Funding; Regeneron: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; Allogene Therapeutics: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding. Orlowski:BMS/Celgene Corporation, CARsgen Therapeutics, Exelixis Inc., Heidelberg Pharma, Janssen Biotech Inc., Sanofi/Genzyme, Takeda Pharmaceuticals USA Inc.: Other: Clinical Research Funding, Research Funding; AbbVie, Adaptive Biotech, Asylia Therapeutics, Inc., BioTheryX, Bristol-Myers Squibb Pharmaceuticals, Karyopharm Therapeutics, Meridian Therapeutics, Monte Rosa Therapeutics, Nanjing IASO Biotherapeutics, Neoleukin Corporation, Oncopeptides AB, Pfizer, In: Consultancy, Honoraria; Asylia Therapeutics: Current equity holder in private company, Patents & Royalties; Asylia Therapeutics, BioTheryX Inc., Heidelberg Pharma: Other: Laboratory Research Funding, Research Funding. Rafei:Takeda: Other: H. R. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical. . Marin:Affimed: Patents & Royalties; Takeda: Patents & Royalties. Champlin:Johnson & Johnson/Janssen: Consultancy; Actinium Pharmaceuticals: Consultancy; Omeros: Consultancy; Kadmon: Consultancy; Arog: Consultancy; Orca Bio: Consultancy; Takeda Corporation: Patents & Royalties; Cell Source: Research Funding. Shpall:Fibrobiologics: Membership on an entity's Board of Directors or advisory committees; Celaid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Affimed: Other: License agreement; NY Blood Center: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: License agreement; Axio: Membership on an entity's Board of Directors or advisory committees; Syena: Other: License agreement; Navan: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees. Rezvani:Affimed: Other: License agreement; Takeda: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal